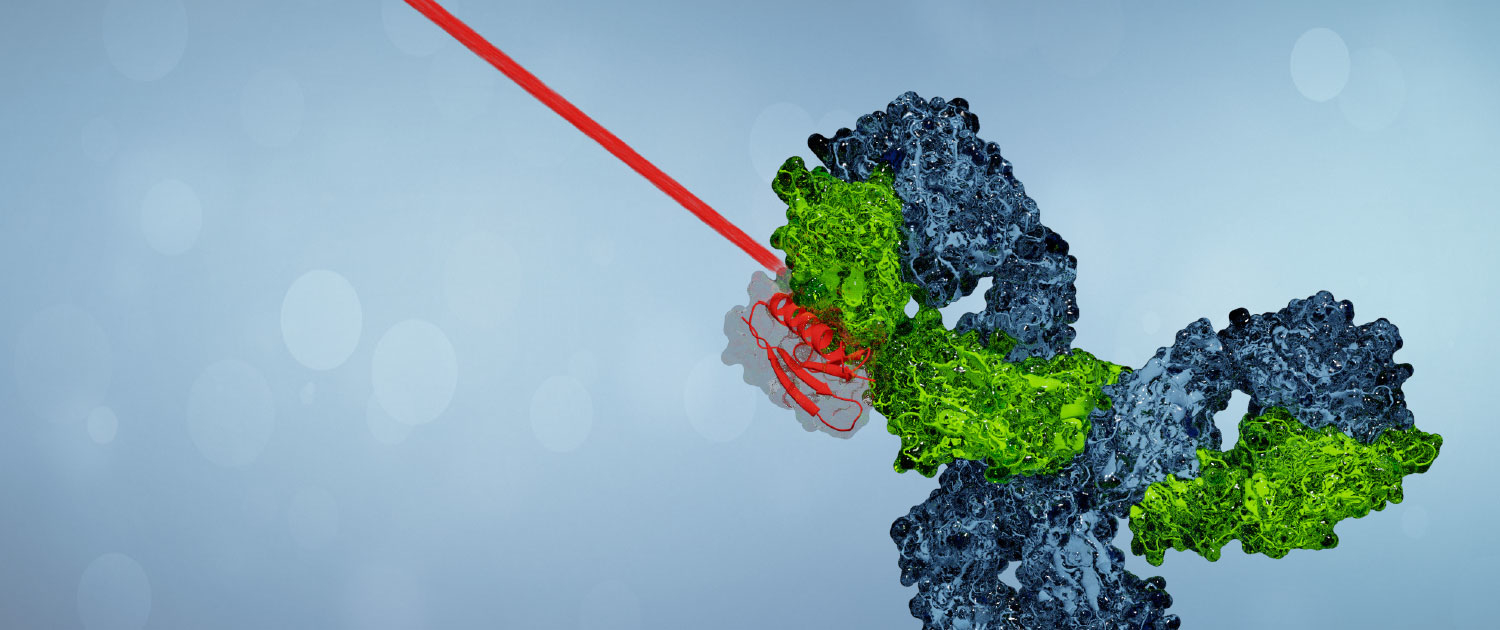
Light Controlled Affinity Matrix
The potential of antibodies for applications in biotechnology and medicine originates from the molecule’s ability to specifically bind specific molecular targets. However, these valuable proteins have to be engineered and manufactured before their use. It is necessary for the researcher, or manufacturer, to isolate the antibodies from a crude extract. A key step in the isolation of antibodies is the well-established affinity chromatography with Protein A resins. The target antibody is eluted from the column using a strong shift in buffer pH to acidic conditions. By this means, antibodies can be isolated to a high degree of purity but at the expense of non-optimal buffer conditions and adverse pH effects on these valuable molecules. These conditions might even exclude sensitive variants entirely from purification.
Despite recent efforts in process development, downstream processing in commercial antibody manufacturing has reached limitations in its linear scalability under today’s demands. In fact, the productivity of Protein A resins has continuously improved but has never been challenged by a fundamentally new technology.
Lumatix Biotech develops a light-switchable affinity ligand for controlled antibody interaction on a solid support.
We have developed an affinity matrix for the isolation of antibodies that is controlled by light. Comparable to traditional methods, antibodies are captured effectively by an immobilized protein ligand. But the affinity towards antibodies can be switched digitally with light of different wavelengths. Lumatix Biotech realizes this disruptive concept by the functionalization of a protein ligand with a proprietary molecular light switch that can change the binding mode: on and off. By this means, antibodies can be isolated in a buffer of choice, comforting the sensitive biomolecules. Furthermore, the mode of operation allows for rapid chromatography cycling with drastically reduced buffer consumption, which implies the potential for huge possible savings. Due to the elimination of a buffer triggered elution step and the ultra-fast regeneration of the affinity matrix after elution, the virtual binding capacity and productivity of the Lumatix chromatography system are superior to common processes.